Double Indicator Titration Lab Report . A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Indicators used in the experiment:. The volumes of acid and alkali solutions that react with each other can be measured by titration using a suitable indicator. Students should be able to: As and a level science. Describe how to carry out. To determine the composition of the following mixture by double indicator method: In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is.
from www.studocu.com
As and a level science. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. The volumes of acid and alkali solutions that react with each other can be measured by titration using a suitable indicator. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. Describe how to carry out. Indicators used in the experiment:. Students should be able to: To determine the composition of the following mixture by double indicator method:
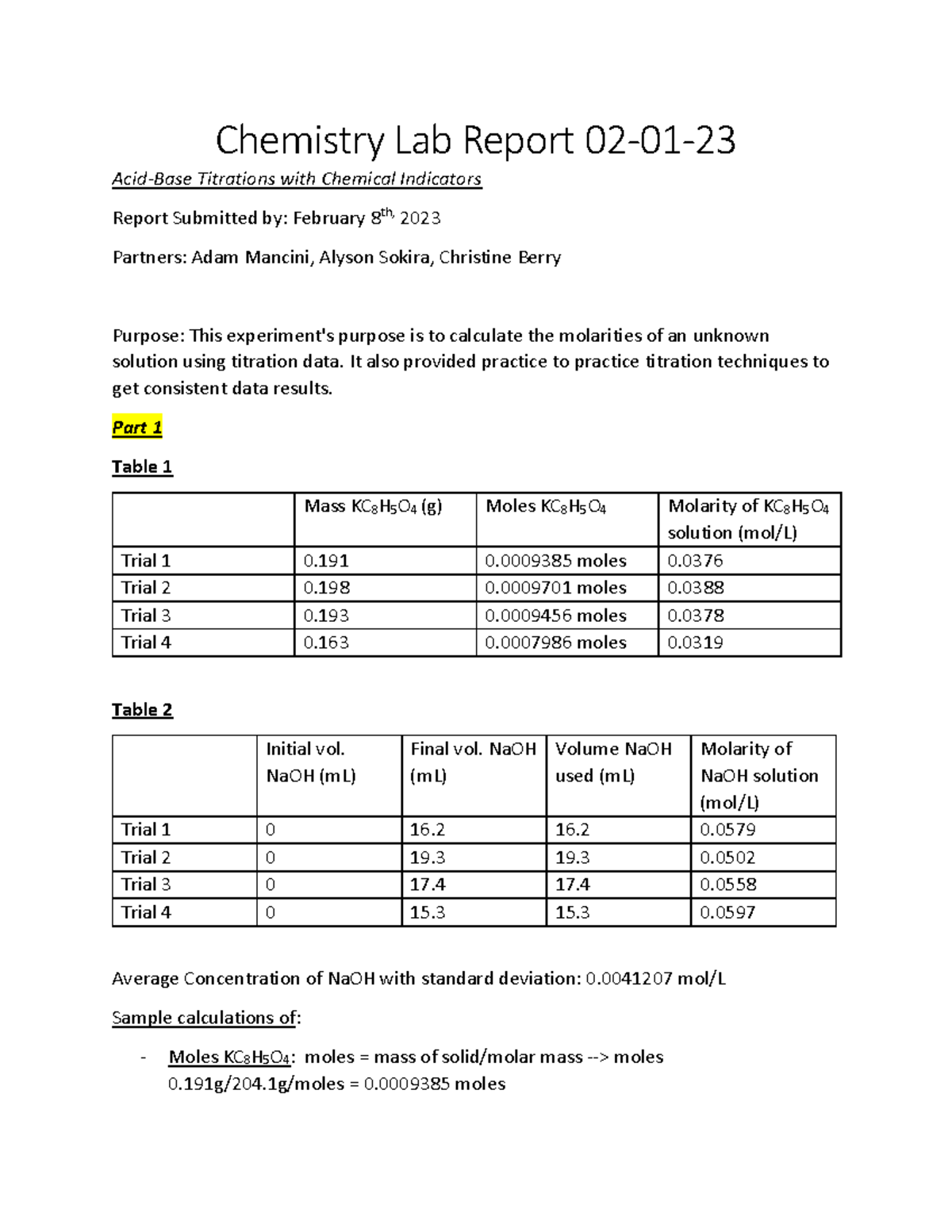
AcidBase Titrations with Chemical Indicators Lab Report 020123
Double Indicator Titration Lab Report As and a level science. Indicators used in the experiment:. As and a level science. Students should be able to: To determine the composition of the following mixture by double indicator method: The volumes of acid and alkali solutions that react with each other can be measured by titration using a suitable indicator. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Describe how to carry out.
From studylib.net
Double Indicator Titration & Solubility Product (Ksp) Double Indicator Titration Lab Report To determine the composition of the following mixture by double indicator method: A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in. Double Indicator Titration Lab Report.
From www.tutormyself.com
(f) Acids, alkalis and titrations Archives TutorMyself Chemistry Double Indicator Titration Lab Report Students should be able to: In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. The volumes of acid. Double Indicator Titration Lab Report.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Double Indicator Titration Lab Report Students should be able to: Indicators used in the experiment:. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. The volumes of acid and alkali solutions that react with each other can be measured by titration using a suitable indicator. As and a level science. In a. Double Indicator Titration Lab Report.
From www.numerade.com
SOLVED During the double indicator titration of a mixture of Na2CO3 Double Indicator Titration Lab Report Indicators used in the experiment:. As and a level science. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Students should be able to: The volumes of acid and alkali solutions that react with each other can be measured by titration using a suitable indicator. In a. Double Indicator Titration Lab Report.
From www.scribd.com
LAB Report 3 Titration Chemistry Double Indicator Titration Lab Report Describe how to carry out. As and a level science. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. Students should be able to: A titration is an analytical procedure used to determine the accurate concentration of a sample by. Double Indicator Titration Lab Report.
From www.science-revision.co.uk
Titrations Double Indicator Titration Lab Report In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. Describe how to carry out. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. To determine the composition. Double Indicator Titration Lab Report.
From www.youtube.com
DOUBLE INDICATOR TITRATION PRACTICAL 01 YouTube Double Indicator Titration Lab Report Indicators used in the experiment:. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Students should be able to: To determine the composition of the following mixture by double indicator method: In a ph titration you measure the ph as a function of the volume of titrant. Double Indicator Titration Lab Report.
From www.slideshare.net
Titration Lab Report Double Indicator Titration Lab Report Describe how to carry out. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. To determine the composition. Double Indicator Titration Lab Report.
From www.scribd.com
The Double Indicator Method Sodium Carbonate Sodium Hydroxide Double Indicator Titration Lab Report A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. The volumes of acid and alkali solutions that react with each other can be measured by titration using a suitable indicator. Students should be able to: To determine the composition of the following mixture by double indicator method:. Double Indicator Titration Lab Report.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Double Indicator Titration Lab Report The volumes of acid and alkali solutions that react with each other can be measured by titration using a suitable indicator. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Students should be able to: As and a level science. To determine the composition of the following. Double Indicator Titration Lab Report.
From childhealthpolicy.vumc.org
😱 Titration lab report discussion. Lab Report 9. 20221018 Double Indicator Titration Lab Report To determine the composition of the following mixture by double indicator method: Describe how to carry out. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence. Double Indicator Titration Lab Report.
From www.studocu.com
Titration Lab Report About the lab tiration Titration Lab Report Double Indicator Titration Lab Report Describe how to carry out. As and a level science. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. The volumes of acid and alkali solutions that react with each other can be measured by titration using a suitable indicator. In a ph titration you measure the. Double Indicator Titration Lab Report.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Double Indicator Titration Lab Report The volumes of acid and alkali solutions that react with each other can be measured by titration using a suitable indicator. Indicators used in the experiment:. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. A titration is an analytical. Double Indicator Titration Lab Report.
From www.studocu.com
AcidBase Titrations with Chemical Indicators Lab Report 020123 Double Indicator Titration Lab Report In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. To determine the composition of the following mixture by double indicator method: Students should be able to: The volumes of acid and alkali solutions that react with each other can be. Double Indicator Titration Lab Report.
From www.youtube.com
DOUBLE INDICATOR TITRATION PRACTICAL 2 YouTube Double Indicator Titration Lab Report The volumes of acid and alkali solutions that react with each other can be measured by titration using a suitable indicator. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. Students should be able to: A titration is an analytical. Double Indicator Titration Lab Report.
From childhealthpolicy.vumc.org
💣 Discussion lab report acid base titration. Lab Report Acid Base Double Indicator Titration Lab Report As and a level science. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Students should be able to: In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there. Double Indicator Titration Lab Report.
From mavink.com
Acid Base Titration Lab Procedure Double Indicator Titration Lab Report Students should be able to: A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. The volumes of acid and alkali solutions that react with each other can be measured by titration using a suitable indicator. To determine the composition of the following mixture by double indicator method:. Double Indicator Titration Lab Report.
From www.scribd.com
Double Indicator Titration Lab Report PDF Double Indicator Titration Lab Report Indicators used in the experiment:. The volumes of acid and alkali solutions that react with each other can be measured by titration using a suitable indicator. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. To determine the composition of. Double Indicator Titration Lab Report.